
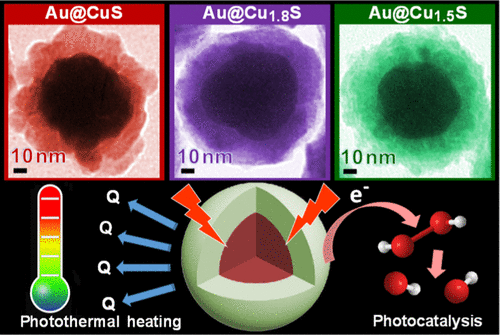
The presence of stable shells provides the unique environment around cores, functioning as microcapsular-like reactors in which adsorption and catalytic reaction are enhanced on the cores. The obtained core-shell catalysts show not only superior activity but also better stability than the naked nanoparticles or the supported counterparts. The core property can also be influenced by core starting material and nominal core/shell ratio. The characterization results indicate that the dimension and dispersion of the core particles is dependent on the core constitution, and the shell encapsulation effect (thickness of shell, single or hierarchical enwrapping) is determined by the nature of cores as well as the core-shell interaction. N2 - The core-shell nanostructures Al2O 3, MgO M = Fe, Co, Ni, Ru) are synthesized and applied in the production of COx-free H2 through NH3 decomposition. T1 - Core-shell structured nanoparticles Al 2O3, MgO M = Fe, Co, Ni, Ru) and their application in COx-free H2 production via NH3 decompositionĬopyright 2010 Elsevier B.V., All rights reserved. The stable shells effectively prevent the core particles from aggregation during reaction.",
The findings presented herein reveal the importance of photophysical and structural properties, such as photocatalyst size and excited state lifetime, in SET and photocatalytic efficiency, thereby contributing to guided optimization of photocatalytic systems.Abstract = "The core-shell nanostructures Al2O 3, MgO M = Fe, Co, Ni, Ru) are synthesized and applied in the production of COx-free H2 through NH3 decomposition. This is attributed to new, less reactive excited states and increased photocatalyst size, which slows down diffusion. The rates of SET and product formation in the trifluoromethylation of quinoline are slower upon incorporation of pyrene. Excited state lifetime and transient absorption studies indicate that only the complex with pyrene separated from bpy by an alkyl bridge displays REET. The excited state lifetimes are 13.8 µs, 4.8 µs and 3.2 µs, respectively. PF₆, PF₆, and PF₆ were prepared where npy = 2-(naphthalen-1-yl)pyridine, dmbpy = 4,4ʹ-dimethyl-2,2ʹ-bipyridine, bpyethylpyr = 4-methyl-4ʹ-2,2ʹ-bipyridine and bpypyr = 4-(1ʹʹ-pyrenyl)-2,2ʹ-bipyridine. The unchanged rate of product formation indicates that SET involving the excited state is not rate-limiting in this system.įurther increase in the SET rate was attempted by attaching pyrene onto a ligand in the complex for Reversible Electron Energy Transfer (REET) to increase the photocatalyst excited state lifetime more significantly. The photocatalyzed trifluoromethylation of quinoline was used as a prototypical reaction.

Their excited state lifetimes range from 0.3 µs to 0.7 µs and correlate to the rate of single-electron transfer (SET) from excited state to substrate, CF₃SO₂Cl (1.9 × 10⁸ M‾¹ s‾¹ to 9.3 × 10⁸ M‾¹ s‾¹), but the rate of final product formation is unchanged. PF₆, PF₆, PF₆, and PF₆ were prepared where ppy = 2-phenylpyridine, bpy = 2,2ʹ-bipyridine, dmbpy = 4,4ʹ-dimethyl-2,2ʹ-bipyridine, dtbbpy = 4,4’-di-tert-butyl-2,2ʹ-bipyridine and phen = 1,10-phenanthroline. Iridium(III) complexes were tailored to increase the excited state lifetime through minor ligand modification. Carbon dioxide was photocatalytically reduced in water by the NPs with methane being the major product. The Pd cores and porous, high surface area TiO₂ shells are expected to prevent Pd loss and increase surface-substrate interactions, respectively, thereby improving photocatalytic efficiency. Core-shell palladium-titanium dioxide NPs, were prepared using monodisperse core-shell NPs as a template. Titanium dioxide nanoparticles (NPs) and iridium(III) complexes have been prepared and studied as photocatalysts towards enhanced efficiency and mechanistic understanding of photocatalysis.


 0 kommentar(er)
0 kommentar(er)
